| Complexity level: | 9 |
| Project cost ($): | 10 |
| Time required: | 1 hour to prepare, 2 days for the science fair project |
| Material availability: | Easily found |
| Safety concerns: | Basic safety requirements |
Hypothesis
Water will pass through the shell of an egg, to the side that has a solution of higher concentration.
Overview
Osmosis
Osmosis is the process by which water travels through a semi permeable membrane from a solution having a low concentration to a solution having a higher concentration. The solution with a low concentration is referred to as a “hypotonic” solution, and the one with a higher concentration is called “hypertonic”. The purpose of this process is to equalize the concentration of the solute on both sides of the membrane.
The process of osmosis is very important for living organisms. Cells use this process to pull in and expel water from within the cell walls. The walls of the cells act as the semi-permeable membrane. The membrane allows smaller molecules like water to pass through but prevents larger organic molecules from leaving the cell.
Plants use this process to absorb water from the surrounding soil into their roots. Their roots normally branch out in search of water and to increase the surface area for absorbing water.
Scientific Terms
Materials
The materials required for this science project:
- 3 eggs
- 1200ml of distilled water
- 300gram of salt
- 1 measuring cylinder
- 3 beakers
- 1 weighing machine
- 1 spatula
- 3 piece of styrofoam cut to beaker size
- 1 black permanent marker pen
Procedure
1. For this science fair project, the independent variable is the amount of salt in the water. The dependent variable is the weight of the eggs before and after being immersed in the solution. This is determined by using a weighing machine to check the weight of the eggs. The constants (control variables) are the amount of water used and the time during which the eggs were immersed in the water.
2. The beakers are labeled A, B and C. 400ml of water is filled into each of the beakers. In the beaker labeled B, 100g of salt is poured in and stirred using a spatula. Likewise, in beaker C, 200g of salt is poured in and stirred using the spatula.
3. The eggs are labeled A, B and C using the marker pen. The weights of the eggs are measured using the weighing machine and recorded in the table given below.
4. Each of the eggs is then placed in beakers labeled A, B and C. The cut styrofoam pieces are placed inside the beakers to force the eggs to remain fully immersed in the water. The beakers and the eggs are put aside for 24 hours.
5. After 24 hours, i the eggs are removed from the beakers and wiped dry. Their weights are checked again using the weighing machine. The new weights are recorded in the table given below.
6. The weight difference of the eggs before they were immersed in the water and after 24 hours is calculated and recorded. The percent of weight difference is then calculated as follows :
% weight difference = (final egg weight – initial egg weight) / initial egg weight x 100%
Results
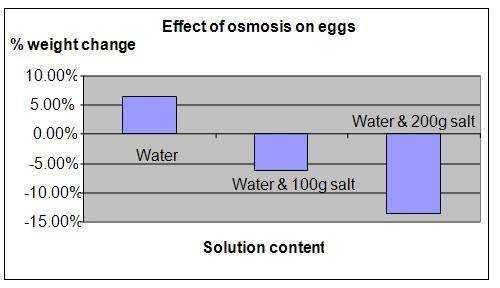
The results show that the weight of the egg placed in distilled water has increased, while the eggs placed in salt water had decreased in weight.
|
Solution |
Initial egg weight (g) |
Final egg weight (g) |
Weight difference (g) |
% weight change |
|
Water |
82.42 |
87.74 |
5.32 |
6.45% |
|
Water & 100g salt |
81.46 |
76.38 |
-5.08 |
-6.24% |
|
Water & 200g salt |
84.73 |
73.29 |
-11.44 |
-13.5% |
The chart below represents the results of our science project experiment.

Conclusion
Our hypothesis has been proven to be correct - water will pass through the shell of an egg to the side that has a solution of higher concentration.
. In the case of the egg in beaker A, water from outside the egg moved into the egg increasing its weight. For the eggs in beakers B and C, the water moved out of the egg, diluting the salt water and reducing the weight of the egg.
The osmosis and reverse osmosis process has found its use in the water purification process. Countries lacking in fresh water resources now use this process for water recycling.
Also consider
The science project can be repeated by using different soluble materials like sugar or syrup.
Repeat the science project experiment at different temperatures to see if temperature has any effect on the osmosis process.
References
Osmosis - http://en.wikipedia.org/wiki/Osmosis
How does reverse osmosis work? - http://science.howstuffworks.com/reverse-osmosis.htm

