| Complexity level: | 8 |
| Project cost ($): | 40 |
| Time required: | 1 hour to prepare, 1 hour for the science project experiment |
| Material availability: | Access to a school's science laboratory is required |
| Safety concerns: | Safety gear/gloves need to be worn at all times when handling acids and other chemical substances |
Hypothesis
All the antacids will neutralize different amounts of acid but Milk of Magnesia will be able to neutralize the most acid.
Overview
Antacids
Antacids are a type of medication that can be purchased at pharmacies to cure heartburn and gastritis. An anticid works by neutralizing the acid in your stomach and increasing pH levels.
Our stomach is normally at pH levels of around 2 to 3. When we eat food, our stomach produces acid to help digest the food. However when we eat to much, our stomach produces too much acid and pH levels may drop below 2. This is when we start to experience heartburn, and that's precisely when the antacids become useful. It helps to bring pH levels back to around 3 to 4, and often offers quick relief.
Antacids will not be able to neutralize the acid in the stomach by itself and will need another ingredient to help increase pH levels. This ingredient can be sodium, magnesium, calcium or aluminum.
Some over-the-counter antacids contain a H2 blocker or Proton Pump Inhibitors. These medications help reduce the amount of acid produced by the stomach. While they may not give immediate relief, and may take more than 1 hour, the relief from the symptoms will last much longer.
Scientific Terms
Materials
The materials required for this science fair project:
- 4 beakers
- 800ml water
- 80ml hydrochloric acid
- 4 brands of antacid – Milk of Magnesia, Gaviscon, Turns, Tagamet
- 1 pH meter
- 1 burette
- 1 bowl and pistle
- 1 glass stirrer
- 1 measuring spoon
- 1 marker pen
Procedure
1. For this experiment, the independent variable is the brand of antacid. The dependent variable is the amount of acid needed to neutralize the antacid. This is determined by measuring the amount of acid dropped from the burette, and by using a pH meter. The constants (control variables) are the concentration of acid solution and the amount of water in the beaker.
2. The 4 beakers are filled with 200ml of water each. Using the marker pen, the 4 beakers are labeled Magnesia, Gaviscon, Turns and Tagamet.
3. The recommended dosages of the 4 types of antacids are mixed together with the water in their respective beakers, named according to the brands. The tablet form of antacid is crushed first using the bowl and pistle before mixing it with water. The liquid form of the antacid is dispensed using a measuring spoon.
4. 40ml of hydrochloric acid is poured into the burette.
5. The burette is placed over the beaker labeled Milk of Magnesia. The acid in the burette is inserted, drop by drop into the beaker. With every drop, the solution is mixed, using the stirrer and the pH reading is taken, until it reaches 7.0. The amount of acid required to neutralize the solution in the beaker is recorded in the table below.
6. The burette is refilled with hydrochloric acid until 40ml again for the next test.
7. Procedures 5 and 6 are again repeated for the beakers labeled Gaviscon, Turns and Tagamet.
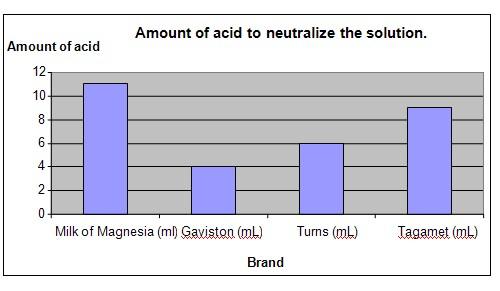
Results
It was observed that Milk of Magnesia was able to neutralize the highest amount of acid and Gaviston neutralized the least amount.
|
Brand name |
Amount of acid needed to neutralize the Antacid solution |
|||
|
Milk of Magnesia (ml) |
Gaviston (mL) |
Turns (mL) |
Tagamet (mL) |
|
|
Amount of acid required |
11 |
4 |
6 |
9 |
The chart below represents the results of our science experiment

Conclusion
The hypothesis that all antacids will neutralize different amounts of acid but that Milk of Magnesia is able to neutralize the most amount of acid is proven to be true. It is no wonder that Milk of Magnesia has remained a popular product for decades.
Antacids are able to give immediate relief from heartburn and gastritis by reducing acid levels in the stomach. However excessive use of antacids has been known to produce side effects such as diarrhea. Patients with kidney problems are advised not to use antacids.
Also consider
What would happen if the experiment were tobe continued until the pH level reached 3, the level in our stomach?
The experiment can be repeated using other brands of antacids.
References
Antacid - http://en.wikipedia.org/wiki/Antacid
Antacid - http://gerd.emedtv.com/antacids/antacids.html

