| Complexity level: | 5 |
| Project cost ($): | 10 |
| Time required: | 1 hour to prepare, 4 hours for observation |
| Material availability: | Easily found |
| Safety concerns: | None |
Hypothesis
When salt is added to water and the temperature of the water increased, the surface tension of water is reduced.
Overview
Surface tension of water
Surface tension in water describes a condition where the surface of water that is in contact with air acts like a thin elastic sheet. It is a phenomenon that occurs on the surface of liquids when they are in contact with a gaseous medium. When there is contact between the surfaces of two liquids like water and oil, it is called interface tension.
The molecules in a liquid (eg. water) are drawn together by intermolecular forces known as Van der Waal forces. The molecules on the surface of the water are not surrounded by water molecules on all sides. They will cohere more strongly with neighboring water molecules, as opposed to air molecules. This creates a “film” on the surface which requires a certain amount of force to penetrate.
The shape that is formed by a drop of water is caused by surface tension. Ideally all liquids will form a spherical shape in the absence of gravity in order to minimize their surface tension. This is because the sphere has the smallest surface area for a given volume. However, the shape of a water droplet is not spherical due to the force of gravity.
Scientific Terms
Materials
The materials required for this science fair project:
- 2 beakers
- 400ml distilled water
- A measurement cylinder
- 2 tablespoons of salt
- A cup of rice grains
- 2 pieces of aluminum foil measuring 1cm x 1cm each
- A refrigerator
- A hot plate
- A thermometer
- A hot plate
Procedure
1. For this science fair project, the independent variable is the salinity of the water and its temperature – 15°C, 25°C, 35°C, 45°C and 55°C. The dependent variable is the weight exerted on the aluminum foil before it sinks. This is determined by gradually adding grains of rice on the surface of the aluminum foil. The constants (control variables) are the size of the aluminum foil, the size of the grains of rice and the room temperature.
2. The 2 beakers are labeled “pure water” and “salt water”. The beakers are each filled with 200ml of distilled water. In the beaker labeled “salt water” 2 tablespoons of salt are added and mixed into the water. The 2 beakers are placed in the refrigerator until the contents in the 2 cups reach 15°C. The beakers are taken out of the refrigerator and the required temperature confirmed using a thermometer.
3. The 1cm x 1cm piece of aluminum foil is made to float on the surface of the water in the beaker labeled “pure water”. The grains of rice are placed one at a time on the aluminum foil until the aluminum foil sinks into the water. The number of rice grains placed on the foil is recorded and the results shown in the table below.
4. Procedure 3 is repeated with the beaker labeled “salt water” and the results recorded in the table below.
5. The 2 beakers are brought to a room temperature of 25°C and Procedure 3 is repeated on these 2 beakers. The results recorded in the table below.
6. The 2 beakers are then placed on a hot plate and heated to temperatures 35°C, 45°C and 55°C. Procedures 3 and 4 are repeated at each required temperature level and the results recorded in the table below.
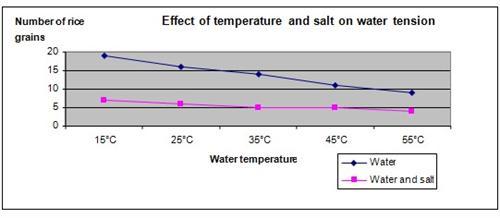
Results
It is observed that when salt is added to water and the water temperature increased, the number of rice grains required to sink the aluminum foil reduces.
| Water solution | Number of grains of rice to sink 1cm x 1cm of aluminum foil | ||||
| 15°C | 25°C | 35°C | 45°C | 55°C | |
| Water | 19 | 16 | 14 | 11 | 9 |
| Water and salt | 7 | 6 | 5 | 5 | 4 |
The graph below represents the results of our science project.

Conclusion
The hypothesis that adding salt to water and increasing the water temperature will reduce its surface tension is correct.
Insects such as the water strider are able to walk on water without sinking. Their legs distribute their weight on the surface of the water so so as to ensure that the downward force applied onto the water surface is not sufficiently large enough for the insect to sink into the water. Similarly, even though paper clips and needles have a higher density than water, they can be made to float on the water surface without sinking because of surface tension.
Also consider
What would happen if the science fair project was repeated with other liquids such as oil or alcohol?
The experiment can be also repeated by adding other substances like sugar or milk into the water.
References
Surface tension - http://en.wikipedia.org/wiki/Surface_tension
Surface tension - http://physics.about.com/od/physicsexperiments/a/surfacetension.htm

