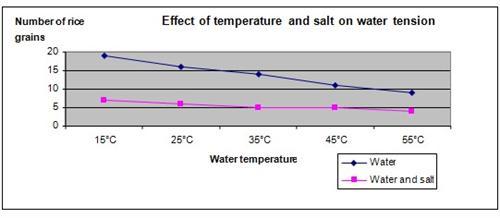
Hypothesis
When salt is added to water and the temperature of the water increased, the surface tension of water is reduced.
Overview
Surface tension of water
Surface tension in water describes a condition where the surface of water that is in contact with air acts like a thin elastic sheet. It is a phenomenon that occurs on the surface of liquids when they are in contact with a gaseous medium. When there is contact between the surfaces of two liquids like water and oil, it is called interface tension.
The molecules in a liquid (eg. water) are drawn together by intermolecular forces known as Van der Waal forces. The molecules on the surface of the water are not surrounded by water molecules on all sides. They will cohere more strongly with neighboring water molecules, as opposed to air molecules. This creates a “film” on the surface which requires a certain amount of force to penetrate.
The shape that is formed by a drop of water is caused by surface tension. Ideally all liquids will form a spherical shape in the absence of gravity in order to minimize their surface tension. This is because the sphere has the smallest surface area for a given volume. However, the shape of a water droplet is not spherical due to the force of gravity.