| Complexity level: | 5 |
| Project cost ($): | 20 |
| Time required: | 1 hour for preparation, 1 hour for experiment |
| Material availability: | Iodine may be obtained from a chemist. Basic laboratory equipment required. |
| Safety concerns: | None |
Hypothesis
The unripe orange will have the highest concentration of vitamin C.
Overview
Oranges and Vitamin C
Vitamin C, or ascorbic acid, is the nutrient found in citrus fruits like oranges, grapefruit and lemons. The high amount of vitamin C in oranges makes it an ideal food choice as our body requires the vitamin. However, vitamin C in oranges degrades over time. Oranges plucked earlier and kept in cold storage contain more vitamin C than oranges left to ripen on the trees. Similarly, freshly-squeezed orange juice is also known to lose its nutrients over time, unless refrigerated. Hence, oranges and orange juice need to be stored in a cool place to retain their vitamin C content.
The use of pesticides and fertilizers on oranges degrades the vitamin C found in the fruit, so pesticide-free produce may be a more nutritious choice. The type of containers used to store fruits can also be important in preserving its vitamin C content, with cardboard boxes lined with foil the best choice for storing oranges.
Scientific Terms
Materials
The materials required for the science fair project:
- 1 ripe orange
- 1 half ripe orange
- 1 unripe orange
- 3 beakers
- 1 juice extractor
- 1 burette
- 1 bottle of iodine - starch solution
- 1 glass stirring rod
- 1 stand and holder to support the burette
- 1 measuring cylinder
Procedure
1. For this science fair project, the independent variable is the type of orange used – ripe, half ripe and unripe. The dependent variable is the number of iodine-starch drops required to neutralize the vitamin C in the juice, determined by dropping iodine-starch into the orange juice until the solution turns blue. The constants (control variables) are the amount of orange juice, the concentration of iodine and the temperature of the environment, which will remain at room temperature.
2. Label the 3 beakers as ripe, half ripe and unripe respectively. Use the juice extractor to extract the juice from each orange. Measure 100ml of each type of orange juice with the measuring cylinder, and pour into the appropriate beaker.
3. Mount the burette on the stand and pour the iodine-starch solution into the burette.
4. Place the beaker of ripe orange juice under the burette. Add the iodine-starch solution to the orange juice one drop at a time, by adjusting the burette. After each drop, the blue iodine-starch will react with the vitamin C and become clear. Once all the vitamin C has neutralized, the color of the iodine-starch will not clear, but instead remain blue. Stop the procedure and record the number of drops taken to neutralize the vitamin C in a table, as shown below.
5. Repeat step 4 with juice from the half ripe and unripe oranges.
Results
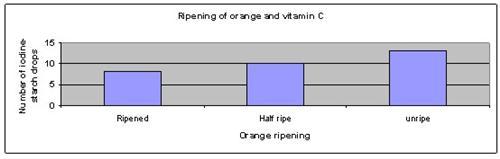
The results show that the unripe orange has the most vitamin C, while the fully ripened orange has the lowest amount of vitamin C.
| State of orange | Ripened | Half ripe | Unripe |
| Number of iodine-starch drops | 8 | 10 | 13 |
The above results were then plotted onto a graph, as shown below.

Conclusion
The hypothesis that the unripe orange will have the highest concentration of vitamin C has been proven to be true.
Vitamin C is an important part of our nutrition and is also an antioxidant. A lack of vitamin C in our bodies causes scurvy, a disease that causes teeth and bone abnormalities. Vitamin C can be found in abundance in fruits and vegetables, which are the main source of the vitamin for most of us. However, it is normally destroyed during cooking. Therefore, it is recommended to eat fruits and vegetables as raw as possible.
Also consider
The science fair project may be repeated by using different types of fruits like papayas or apples.
The experiment may also be repeated by leaving the freshly squeezed juice in the open for a period of time, before testing for the gradual degradation of vitamin C.

