| Complexity level: | 7 |
| Project cost ($): | 20 |
| Time required: | 1 hour to prepare, 1 hour for this science fair project |
| Material availability: | Easily available at a hobby store |
| Safety concerns: | Use only batteries. Do not connect the wires to a mains supply. |
Hypothesis
A higher DC voltage applied to the electrolysis process will increase the rate at which hydrogen gas is produced. The second hypothesis is that the rate of production of hydrogen gas will increase as the concentration of electrolyte salt is increased.
Overview
Electrolysis process
The electrolysis process is a chemical reaction that takes place in an electrolyte solution when electricity is passed through it. The electrolyte solution contains both positive and negative ions. The positive ions are called anions while the negative ions are known as cations. Both the anions and cations will move freely inside the electrolyte solution.
Conductors, called electrodes, are connected to the battery terminals and immersed in the electrolyte solution for the electrolysis process to take place. The cathode is the electrode that is connected to the negative terminal of the battery. The anode is the electrode connected to the positive terminal of the battery.
When the terminals of the battery are connected to the electrodes and immersed in the electrolyte, anions will move towards the anode while cations will move towards the cathode.
When the ions reach the surface of the electrodes, the following reactions may happen:
a. Deposits may form on the electrode surfaces.
b. Gases may be released from the electrodes.
c. The deposits and gases formed, may subsequently be dissolved in the electrolyte.
Scientific Terms
Materials
The materials required for this science fair project:
- 1 beaker
- Tap water
- 200 milligrams of salt
- 1 stirrer
- 4 x 1.5V batteries and battery holders
- 2 jumper wires with crocodile clips at both ends
- 2 test tubes
- 2 PVC (polyvinyl chloride) insulated copper wires (5mm2), each measuring 150 millimeters in length
- 1 digital weighing scale
- 1 stopwatch
- 1 pair of pliers
- 1 black permanent marker
Procedure
1. For this science fair project, the independent variables are the DC voltage and the concentration of salt in the electrolyte. The dependent variable is the time taken to collect the released hydrogen gas, and will be measured with the stopwatch. The constants (control variables) are the amount of oxygen collected in the test tube and the surface area of copper wire exposed to the electrolyte solution.
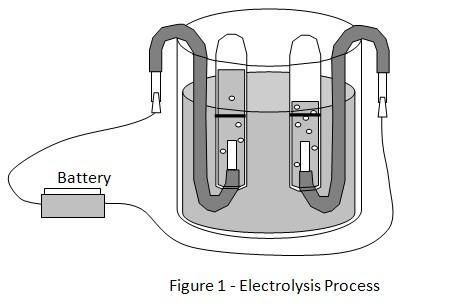
2. Using the pliers, strip both copper wires of approximately 30 millimeters of insulation on both ends. Bend the wire into ‘S’ shapes and place them inside the beaker, as shown below in Figure 1.
3. Fill the beaker with 300 milliliters of water and add 20 milligrams of salt. Stir to dissolve the salt in the water.

4. Draw a line using the permanent marker around the middle of each test tube, along its circumference. Fill the 2 test tubes with water and place them upside down inside the beaker, as shown in Figure 1. The exposed copper wire should be positioned inside the mouth of the test tube.
5. The test is started using one 1.5V battery. Using the jumper wires, connect the battery terminals to the copper wire hanging outside the beaker. Start the stopwatch immediately and record the time taken for the gas collected to reach the black line.
6. Repeat steps 3 to 5 by adding 40 mg, 60 mg and 80 mg of salt into 300 ml of water each time. Record the time taken for the hydrogen gas collected to reach the black line, for the different concentrations of salt.
7. Again, repeat steps 3 to 5, this time, using 2 batteries (3V), 3 batteries (4.5V) and 4 batteries (6V). The concentration of salt in the electrolyte should be kept constant, at 20 mg of salt to 300 ml of water. Record all measurements in a table, as shown below.
Results
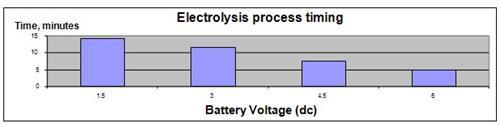
The results show that increasing the DC voltage will result in a faster release of gasses from the chemical reaction in the electrolyte. However, increasing the amount of salt in the electrolyte does not change the rate of gas released from the electrolysis process.
| Salt amount in electrolyte (in mg) | 20 | 40 | 60 | 80 |
| Time measured (in minutes) | 14.4 | 14.3 | 14.3 | 14.4 |
| Voltage applied (V dc) | 1.5 | 3 | 4.5 | 6 |
| Time measured (in minutes) | 14.2 | 11.6 | 7.5 | 4.8 |

Conclusion
The hypothesis that a higher DC voltage applied to the electrolysis process will increase the rate at which the hydrogen gas is produced has been proven to be true.
The second hypothesis, that the rate of production of hydrogen gas will increase as the concentration of salt increases, is not proven to be true.
Electroplating is an industrial process which is used to produce a coating on metal surfaces. Electrolysis is also used in measuring devices such as the pH meter, while astronauts produce oxygen in space through the electrolysis.
Also consider
This science fair project may also be repeated by using different types of electrolytes such as vinegar or sulfuric acid.
Also, examine the effects of raising or lowering the temperature of the solution during the process of electrolysis.
References
Electrolysis - http://en.wikipedia.org/wiki/Electrolysis
Electrolysis - http://www.tutorvista.com/content/chemistry/chemistry-iii/redox-reactions/electrolysis.php

